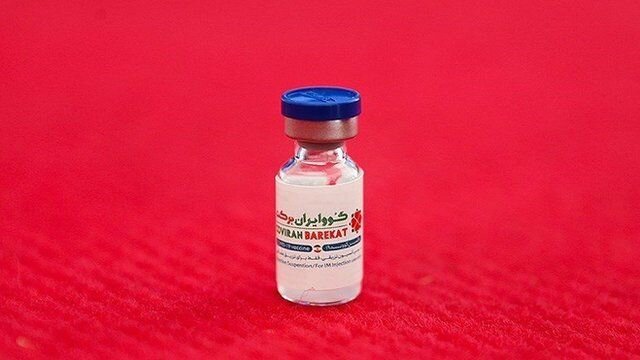
TEHRAN – COVIRAN BAREKAT, the first Iranian-made coronavirus vaccine, is in the process to be registered by the World Health Organization, Asghar Abdoli, an expert in the vaccine production project, has stated.
The whole process of the vaccine development and clinical trials must be presented in detail from the beginning, which we are doing, he said, adding, “in addition to presenting the documents, we must also defend our scientific achievements in person.”
“Our first article on the pre-clinical phase of COVIRAN vaccine will be published soon.”
Vaccine development technical knowledge is native to the country. In other words, the whole process is carried out in Iran, he noted.
He went on to say that some 2 million doses of the vaccine will be produced by the next week, and the production process will continue.
Made by researchers at the Headquarters for Executing the Order of the Imam, COVIRAN BAREKAT was unveiled on December 29, 2020, and received the license for public use on June 14.
It proved effective against Indian strain, according to Hojjat Niki-Maleki, head of the information center of Headquarters for Executing the Order of the Imam.
Eleven countries from Asia and South America, and a European country have asked for importing COVIRAN vaccine, Hassan Jalili, the vaccine’s production manager, has said in June.
Mass vaccination against COVID-19 started on Iranian citizens with the Russian-made Sputnik V vaccine on February 9.
While Iran continues efforts to mass-produce local candidates, 13 million doses of foreign vaccines have already been imported and others are expected soon.
Iran is also producing vaccines jointly with two countries Russia, and Australia, which may also be released by September.
According to the Food and Drug Administration, 14 vaccines are being domestically developed in the country which are in different study phases.

No comments:
Post a Comment