As the number of coronavirus cases around the world surpassed 20 million mark, scientists and pharmaceutical industries around the world are racing towards developing the vaccine for the infectious Covid-19 disease that has caused 772,000 deaths along with 13.5 million recoveries.
Russian President Vladimir Putin announced on August 11 the news of the first vaccine to treat Covid-19. World’s first coronavirus vaccine was developed following trials conducted by the Gamaleya Institute for Epidemiology and Microbiology in Moscow in collaboration with the Defense Ministry.
Putin said one of his daughters has already received dose of the vaccine, which has been named Sputnik-V, referring to the satellite Russia launched into space during the Cold War, beating the U.S. to the punch.
Alexander Ginzburg, a microbiologist, and director of the state-owned Gamaleya Institute of Moscow, injected himself along with one hundred institute employees with the vaccine he developed four months ago. Everyone is still healthy. The Russian government is hoping to approve Ginzburg’s vaccine as rapidly as possible.
Worth mentioning is that Russia approved mass production of the vaccine without undergoing Phase III trials. According to historyofvaccines.org, vaccine development is a long, complex process, often lasting 10-15 years and involving a combination of public and private involvement.
The argument for Putin’s decision could be that since Phase I and Phase II trials have undergone without any safety concerns, the longer-term Phase III trials can be held simultaneously along with general public vaccination. This way thousands of lives could be saved if Russia’s Phase III trials finish without safety concerns.
For many this is a big ‘if’ in the sentence and Putin has been criticized for side-stepping Phase III and approving mass manufacture of the vaccine. As the Persian saying goes: “They sowed the seed of ‘if’ and nothing grew”.
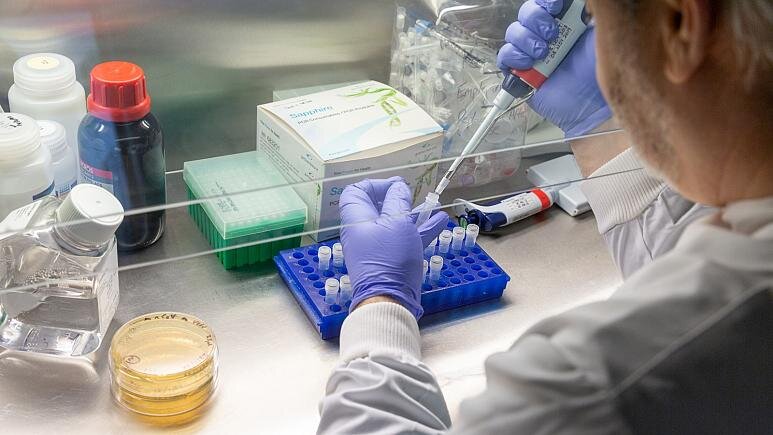
Vaccine development
According to The New York Times, researchers around the world are developing more than 165 vaccines against the coronavirus, and 31 vaccines are in human trials. The report adds that typically vaccines require years of research and testing before reaching the clinic, but scientists are racing to produce safe and effective vaccine by next year.
Hundreds of millions of eggs – the traditional medium for incubating the viruses before they are inactivated and made into vaccines – are delivered to laboratories, and production continues throughout the spring until the syringes are filled in the summer.
The procedure of vaccine testing before final approval is divided into five steps: Stage 1, Phase I, II & III, and Stage 2. The first clinical trials started in March, and now there are some 30 underway.
Before human trials testing the coronavirus, vaccine began with injection into mice or monkey to see how they respond. After animal tests turned out well, researchers enlisted volunteers for a Phase I trial.
Scientists give the vaccine to a small number of people to test safety and dosage as well as to confirm that it stimulates the immune system.
In this phase the volunteers are kept under observation to watch for negative reactions, and to see whether they make antibodies against a pathogen. If Phase I trials go without serious safety problems, a larger group of hundreds of people are registered for Phase II testing.
Scientists give the vaccine to hundreds of people split into two groups, such as children and the elderly, to see if the vaccine acts differently in them.
The goals of Phase II tests are to study the candidate vaccine’s safety, immunology, proposed doses, schedule of immunization, and method of delivery.
Successful Phase II candidate vaccines move to larger and longer trials in Phase III, involving thousands of people. Phase III goal is to assess vaccine safety in a large group as certain side effects might not surface in smaller groups tested in earlier phases. To detect a significant difference for a low-frequency event related to candidate, the trial would have to include 60,000 subjects.
Vaccine efficacy is tested as well as other factors include: 1) Does the candidate vaccine prevent disease? 2) Does it prevent infection with the pathogen? 3) Does it lead to production of antibodies or other types of immune responses related to the pathogen?
After successful Phase III trials the vaccine developer will submit an application for license for mass production. Then the regulating body will inspect the facilities to review the manufacturer’s tests of lots of vaccine for potency.
All participants in the trials must be voluntary and normally receive monetary compensation, but it tends not to be particularly generous. All data from the testing must be made public so government agencies and other scientists can evaluate it.
According to the World Health Organization (WHO), laboratories in 12 countries have developed 27 vaccine candidates, which are now undergoing clinical testing. India and Russia have begun the process of clinical evaluation the purpose of which is to evaluate the clinical handleability, acceptability and delivery.
The Euronews reports of record flu vaccine doses produced by global manufacturers for the upcoming flu season, as authorities hope to avert a crisis in hospitals by a winter rebound of Covid-19.
There are also hopes that measures such as wearing masks and maintaining social distancing will reduce the spread of flu.
With 5.37 million cases and 169,000 deaths, the U.S. has the highest number of infections in any country. Americans are already among the world’s most vaccinated against the flu: vaccination is recommended from the age of six months, while other countries, including France, recommend it for people at risk of complications including those over 65.
In the Persian Gulf region testing for phase III have already begun in UAE and Saudi Arabia in conjunction with Chinese pharmaceutical companies, Sinopharm GNBG and CanSino Biologics respectively.
Circling back to Iran, Mohammad Mokhber, director of the Headquarters for the Execution of Imam Khomeini’s Order, told reporters on August 9 that the first phase of Covid-19 vaccine’s clinical trials is set to begin within the next two weeks.
Minister of Health and Medical Education Saeed Namaki said in June that Iran has taken effective steps towards manufacturing vaccine and human trials will start soon. After successful animal tests human trials were to start soon and Iran could meet the country’s needs
for equipment within two months adding that the country has now become exporter of that equipment for countering Covid-19.
More recently, Namaki said that 98 percent of medical needs are being met indigenously as 200 pharmaceutical companies are active in this area. Iranian officials have announced that the country has exported cargoes of serology test kits, used to identify the presence of antibodies, to Germany and Turkey.
for equipment within two months adding that the country has now become exporter of that equipment for countering Covid-19.
More recently, Namaki said that 98 percent of medical needs are being met indigenously as 200 pharmaceutical companies are active in this area. Iranian officials have announced that the country has exported cargoes of serology test kits, used to identify the presence of antibodies, to Germany and Turkey.
Meanwhile, Hossein Modarres-Khiabani, the caretaker of Iran’s Ministry of Industry, Mine and Trade has said that the country has now become an exporter of facial mask-producing machinery, underlining the importance of the issue after the Supreme Leader of the Islamic Revolution Ayatollah Seyyed Ali Khamenei has named this Iranian calendar year 1399 (March 21, 2020-March 21, 2021) as the year of “Surge in Production.” Iran has a production capacity of 11 million masks per day.
In a press conference held on Sunday Iran’s Health Ministry spokeswoman said that 147 more patients have died of the novel coronavirus in the past 24 hours, increasing the overall death toll to 19,639, reports Tasnim news agency.
In the press briefing, Sima-Sadat Lari confirmed 2,133 new cases of Covid-19 infection, raising the total number of infections to 343,203. She added that 297,486 patients have so far recovered, but 3,881 still remain in critical conditions of the disease.
Lari added that so far 2,861,825 Covid-19 tests have been conducted across the country.
She said the high-risk “red” zones include Tehran, Qom, Mazandaran, West Azerbaijan, Qazvin, Chaharmahal and Bakhtiari, Kohgiluyeh and Voyer-Ahmad, and Zanjan provinces.
Returning back to the topic of vaccine development, if a Covid-19 vaccine is developed what is next? Everyone in the world will need to be vaccinated and there will be a question of distribution. Will there be enough vials to contain the vaccine and enough administering tools? Developing people’s trust in the remedy will be another issue.
TAGS

No comments:
Post a Comment